Overview of U.S. FDA
The U.S. FDA is a government department of the United States Food and Drug Administration. It ensures Health and Human Services. On the other hand, Public health has been given wider attention at this point of time by FDA. This is to meet the current lifestyle of the public and innovation in the nation. Thus U.S. FDA's main purpose is to protect and promote public health and its safety at the initial point. The U.S. FDA came into force in the year 1906. Thus it is regarded as one of the old departments of the Food and Drugs Administration in the nation. And it is mandatory to obtain for the business entity that wants to export any food or drug-related commodities into the U.S.
U.S. FDA has vital importance in supervising the safety measures for consuming food, drugs, medical gadgets, medicines, veterinary products, etc in the US. All these shall be maintained and manufactured as per the guidelines of the FDA.
What does U.S. FDA Certification mean?
U.S. FDA Certification certifies that the product imported to the U.S. by the certificate holder is safe for use. It further provides clarification for the goods and products that they meet the FDA guidelines as prescribed by the concerned administration. Thus all the entities engaged in processing, packaging, and re-packaging of foods, drugs, cosmetics, etc shall obtain the certificate of the U.S. FDA to market such products in other nations.
The Certification helps the Indian market to boost its foods and drugs product into the U.S. market. It also ensures the standard and quality of the products exported. The concerned officials inspect the plants of foods and drugs in India. After inspecting the authority issue Form 483 to the manufacturer for obtaining U.S. FDA Certification.
Role of U.S. FDA
Following is the role of the U.S. FDA:
- It ensures that goods or products such as food, drugs, medications, natural goods, clinical instruments, beauty care products, and other products or goods which produce radiation are safe enough to be consumed or used by the citizens.
- U.S. FDA has mandated the registration for all the packaging, and re-pack of items imported to the US.
- It ensures the nation’s overall food supply.
- It ensures standard, quality, and regulation maintenance of various food and drugs.
- Regulating rules and regulations for all the goods which require approval for export to the US.
- Maintain the product and regular innovations to it as per human health.
Products that require U.S. FDA Approval
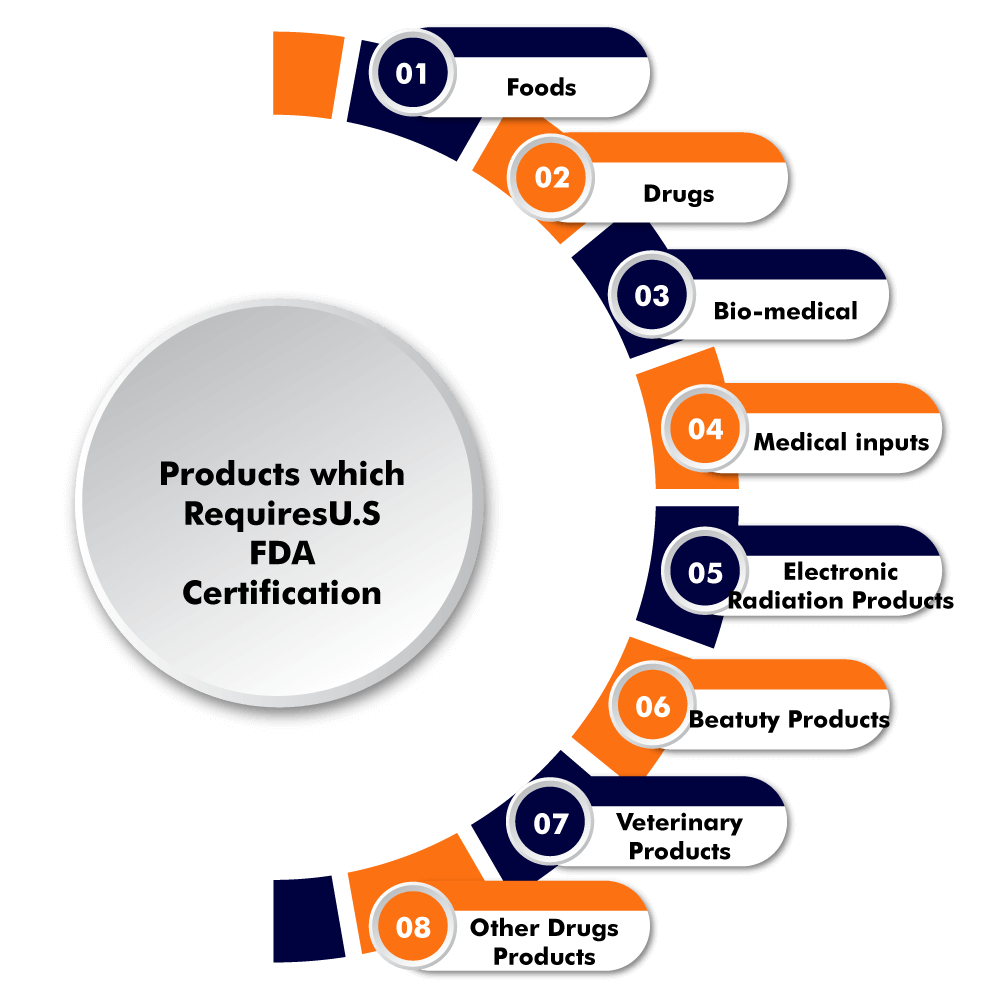
U.S. FDA has certified some products which require prior approval for exporting into the US. Although not all the products require approval and that is dependable on the type of product, one is trying to export in the US. And not all the products require obtaining approval but some just need to get registered with FDA. The following are products require approval for U.S. FDA are mentioned as following:
Foods,which also include the following;
- Water Bottles
- Dietary supplements plans
- Food additives
Drugs, which include the following;
- Drugs are prescribed by the U.S. FDA
- Other non-prescribed drugs
Bio-medical includes the following;
- Human vaccines
- Different types of blood groups
- Genes therapy products
- Cellular therapy products
- Tissue and its different products
- Allergies medications, etc.
Medical Inputs [devices] includes the following;
- Heart pacemakers
- Dental devices
- Surgical implants
- Prosthetics
- Tongue depressors, bedpans, etc
Electronic Radiations productsinclude the following;
- Electronic items
- W-ray equipment
- Laser products
- Sun lamps
- Mercury vapor lamps
- Ultrasonic instruments, etc
Beauty productsinclude the following;
- Skin cleansers, nourishment lotions, moisturizers
- Cosmetics products
- Makeup products
- Other beauty products
- Perfumes,
- Nail paints and remover, etc.
Veterinary productsinclude the following;
- Veterinary foods
- Its various drugs and devices used
- Livestock feeds
Other Drugs productsinclude the following;
- Cigarettes
- Tobacco
- Cigars
- Hookah
- Nicotine
- Other such related products.
Benefits of U.S. FDA Certification
The one who is obtaining the U.S. FDA Certification obtains various benefits in the field. The following are some of the benefits an applicant will receive after obtaining the Certification:
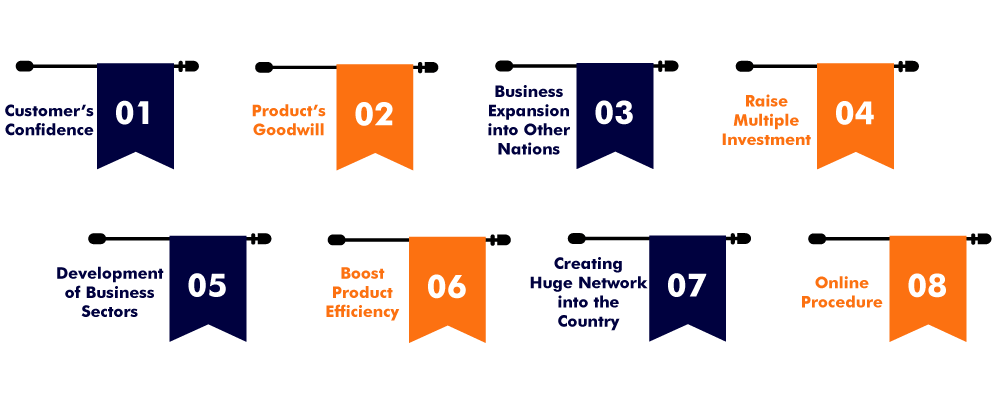
- Customers confidence:
- Products goodwill:
- Business expansion into other nations:
- Raise multiple investments:
- Development of business sectors:
- Boost product efficiency:
- Creating a huge network in the country:
- Online Procedure:
The entity which obtains the U.S. FDA Certification gains the customer’s satisfaction for the products it provides services. Obtaining the certification is not an easy task to be accomplished thus in the end when the same is obtained provides a wide range of customer satisfaction and confidence in the use of products.
The food and Drugs Administration has a rigorous process to be followed due to its international validation. Thus products that attain U.S. FDA Certification have produced goodwill in the market. This results in the rise of demand for products in the market. Thus the Certification is helpful.
The U.S. FDA Certification helps the business to expand further into various other countries. It is an international certificate and not valid in the US only. It includes other nations such as Japan, Australia, China, etc. Thus obtaining one certificate, the doors of business towards other nations is also opened up.
U.S. FDA Certification provides product demand and results in business expansion. And through this, the other entity is likely to invest in the company or its products. Thus in this way the company is likely to raise investments from multiple sources for the company.
The U.S. FDA Certification once obtained will help the business to grow and expand the other areas into the business easily. Further, it also requires regular updating, innovation, and enhancement of the products to maintain its name in the market.
Products efficiency shall be checked and upgraded regularly once the product has obtained the certification from U.S. FDA. Later, its proper testing, production, manufacture, design; etc shall be checked in proper time. This will help the company and the product to be in a stable position in the market.
The products certified by U.S. FDA have a distinct identity in the country. And thus it creates a different network of products in the market due to which many small businesses or entities engage in the network of the product. Thus U.S. FDA Certification leads to create a network in the market due to its goodwill.
Obtaining the U.S. FDA Certification has been made an online process also. It helps an individual seated anywhere to apply for their certificate conveniently. Although due to many formalities, documentation, and procedures, a Professional may be consulted once.
Importance of U.S. FDA Certification in India
U.S. FDA Certification has vital importance in India. The following are some of the reasons supporting the importance of the Certification in India:
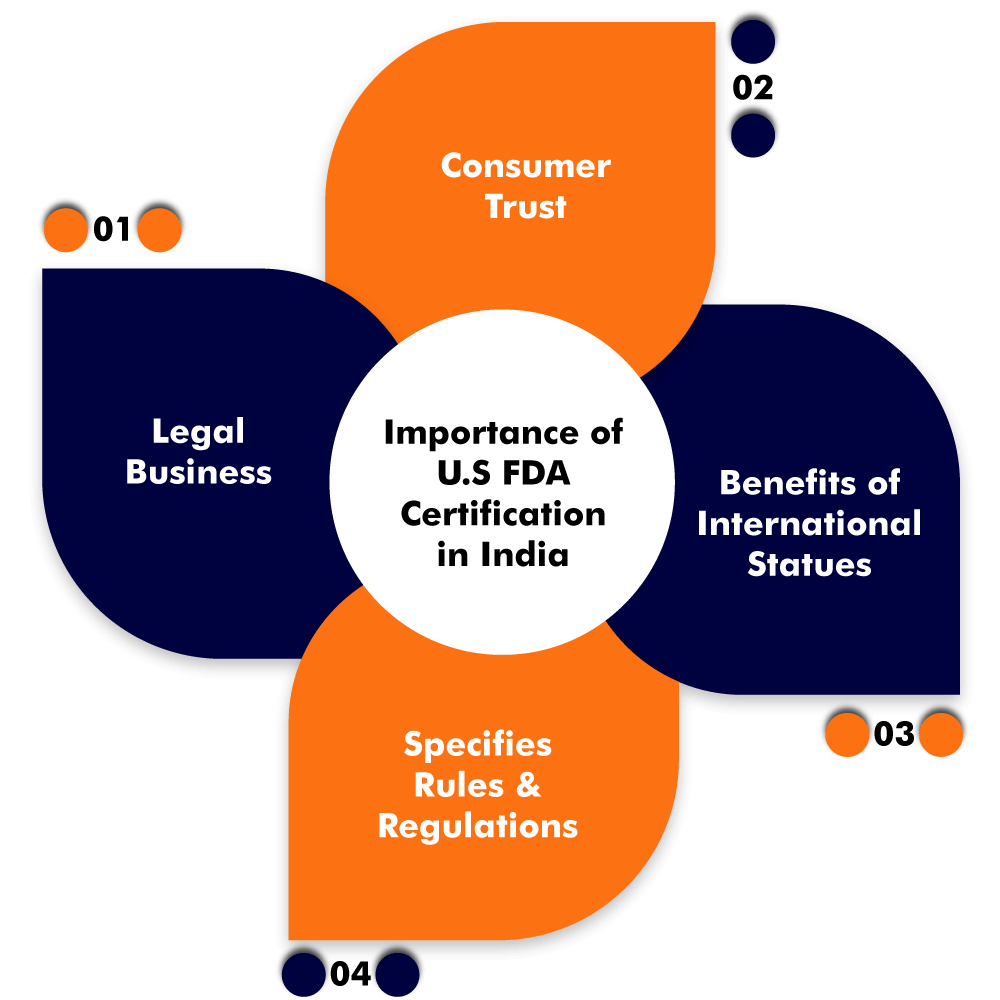
- Legal Business:
- Consumers Trust:
- International benefits of Statutes:
- Specifies Rules and Regulations:
Obtaining the U.S. FDA Certification in India helps the business to run legally in the US and other various nations also. The certificate certifies legal matters regarding the products and their business in the country.
The consumers in India lay down trust in the product certified by U.S. FDA. Due to its goodwill, the marketing of the products is also increased in the country.
The U.S. FDA Certification is an international certificate thus there are various other benefits concerning its which are guaranteed to the compliance of U.S. FDA Certification.
The U.S. FDA Authorities prescribe various rules and regulations and other compliances related to Food, Drugs, cosmetics, medical appliances, etc. This helps the applicant for U.S. FDA Certification to comply and produce its output efficiently in return and boosts the product's goodwill at the international market level.
Activities of the FDA in India
In India, the FDA usually is engaged in regulatory decisions regarding various products which are being exported to the US from India. It also performs various other activities that are mentioned as follows:
- Regular inspections of medical products and equipment that are exported to the US.
- The U.S. FDA also consults and builds relations with Indian regulatory authorities to maintain the required quality and standards of products exported.
- Providing training to the Indian employers and regulators for Indian pharmaceutical and foods manufacturing industries to maintain the quality and standards requirement of foods and drugs.
Prerequisites of U.S. FDA Certification
There are some important key aspects that must be taken into consideration before applying for U.S. FDA. The following mentioned are some essential points:
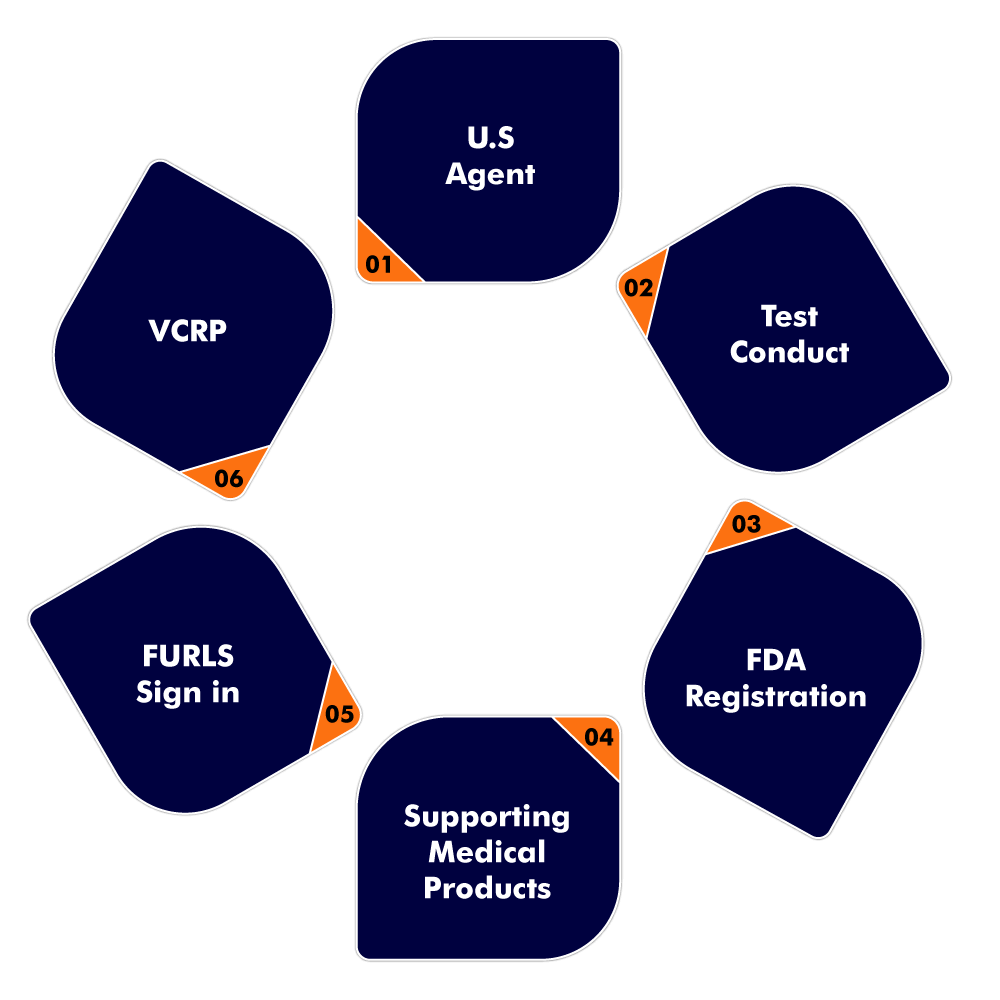
- U.S Agent:
- Test conduct:
- FDA Registration:
- Supporting medical products:
- FURLS:
- VCRP:
Before applying for U.S. FDA, it is essential to appoint a U.S. FDA official in the US on regular basis for legal matters such as sending and receiving notices, responses to notices and other matters, and timely communications when required related to FDA.
All the products which are exported to the US are essentially required to conduct various tests and experiments on the products to satisfy the product safety, quality, and terms of use. All these reports of experts shall be submitted to the authority in charge while obtaining the registration.
All the entities which are engaged in manufacturing, packaging, and marking any product or goods are required to obtain FDA Registration. The entity may be a national or international platform.
All the products exported shall present some proof of documents supporting the medical products exported to the US. This is asked to showcase to maintain the records of medicines exported to the US.
FURLS is FDA Unified Registration and Listing Systems. It is an electronic form in which all the data related to FDA is stored. The applicant shall enlist the entity into FURLS. Thus it would help the applicants to apply online and documents submitted online. It is also used by FDA officials for registration and listing down.
VCRP is the Voluntary Cosmetic Registration Program for FDA. It is a post that advertises in the US for all those who are engaged in cosmetic manufacturing, dealers, packaging, etc.
Documents required for obtaining U.S. FDA Certification
To acquire the U.S. FDA Certification, the following documents are required to be uploaded by the applicant:
- Landing bill
- Airway bill
- Invoices issued by the entity
- Purchase order details
- Packing list details
- Growers list details
- Labeling copies details
- Proof indicating the product’s real owner
- Details showing the use of the product exporting
- Other related documents are requested to showcase.
Procedure for obtaining the U.S. FDA Certification
Follow the below-mentioned steps to obtain the U.S. FDA Certification in India:
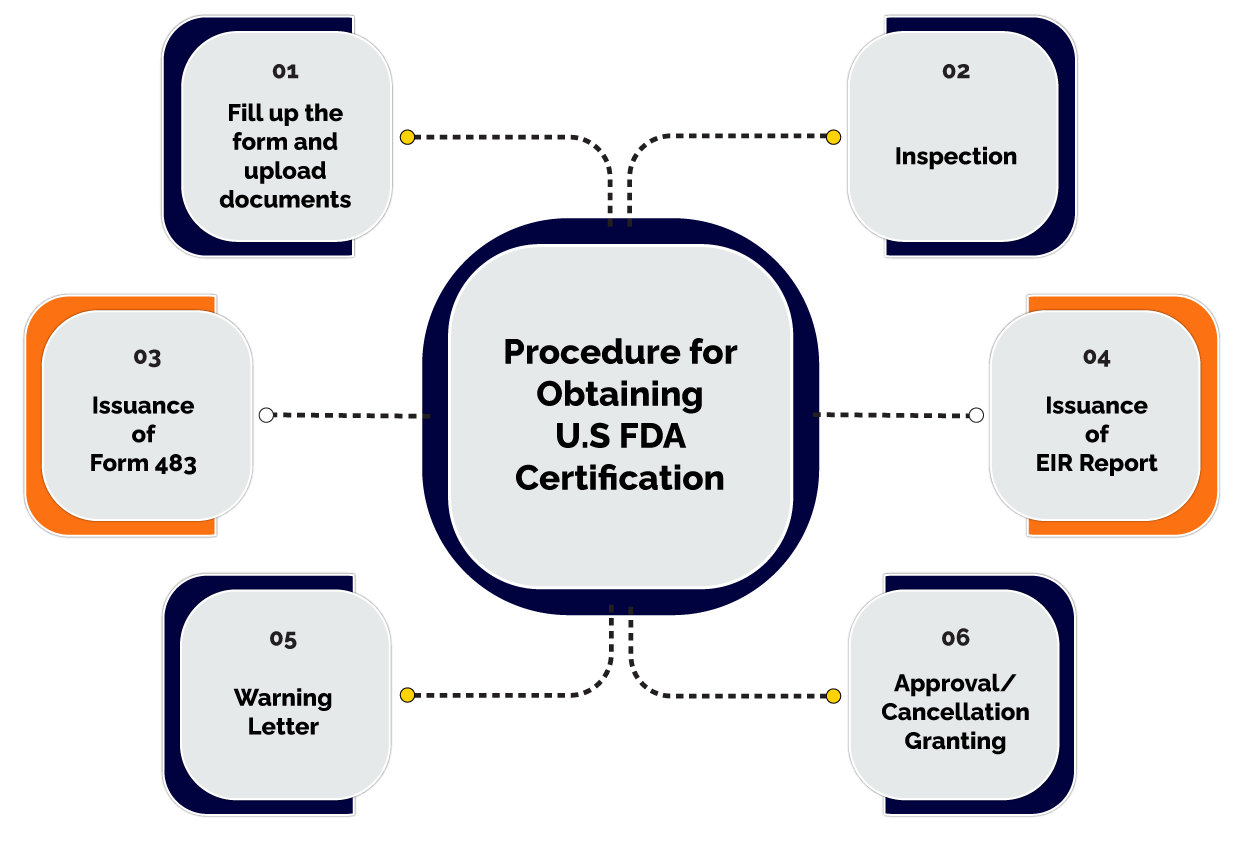
- Fill up the form and upload the required documents:
- Inspection:
- Issuance of Form 483:
- Issuance of EIR Report:
- Warning letter:
- Approval or cancellation granted:
FURLS is an online electronic form in which the applicants need to fill up the form and enter all the required essential details in it regarding the entity, its owner, products registration, its U.S. agent details, etc. after entering the details and validating the same, the applicant is required to upload the documents as proof in the form itself.
After filing the form and uploading the documents, all these are examined by the authority in charge of it. And later the officials concerned are also required to inspect and visit the food and drugs in the US.
After completing the inspection procedure, the concerned officials issue form 483 to the manufacturers. Any deviations found while the inspection shall be mentioned in the form itself.
The reply shall be sent back to the FDA officials within 15 days mentioning the reasons in it for lacking down in any area for inspection. The response shall mention the new action plan supporting the shortcomings in that area.
The FDA officials may also issue Establishment Inspection Report. This report specifies that is there any need for action required to be taken by the entity or not. The action shall relate to pre-clinical testing, investigation of new applications, and review by the FDA.
After receiving the reply to Form 483, if the officials are not satisfied with the response, the officials may issue a warning letter to the manufacturer stating the area of non-satisfaction.
The reply to the warning letter shall be sent within 15 days of issuance of the warning letter to the manufacturer. Now the reply shall be in such a way that the officials are satisfied to issue the U.S. FDA Certification.
When the officials are satisfied with the response of the manufacturer, it issues the applicant the U.S. FDA Certification. The certificate could also be obtained in the initial procedure if the official is satisfied at that point of inspection.
The officials can also cancel the Certification application after a non-satisfactory response is received after the issuance of a warning letter to the applicant.
There may also be chances that after visiting or inspecting the applicant's products or other areas of inspection, on the nonsatisfaction of FDA officials, he or she may directly issue the warning letter to the applicant.
Validity of U.S. FDA Certificate
The U.S. FDA Certificate once obtained by the applicant is valid for 24 months from the date of issuance of the certificate by the concerned authority i.e. CDER. After its validity ends the applicant shall apply for it again via the same process. This is due to non-re-issuance of the certificate.
The Certification is also liable to be canceled or revoked if the authority deems so at any point in time, also that is done so by prescribed procedures. Once the Certification is granted, the related compliances must be followed and maintained by the applicant on regular basis along with the product's safety. And in absence of it, the certificate could be canceled.
Fees to obtain U.S FDA Certification in India
The fee to obtain U.S. FDA Certification in India may vary from office to office and state to state. The application fee varies from Rs. 1500 to 500/- in India depending upon the type of application and the products.
Bizadvisors Assistance for U.S. FDA
Bizadvisors is one of the leading cloud-based technology company which provides legal services and many more related services. We provide U.S. FDA Certification with a minimum time and cost. The services we provide for U.S. FDA Certification are as follows:
- We provide our best Professionals to guide you for U.S. FDA Certification.
- You just need to book an appointment with us and our experts will help you out with your query related to U.S. FDA Certification
- You need to submit the required documents and information for U.S. FDA Certification and our professional will prepare the application in the prescribed format and will guide you for further compliances and requirements for it.
- We apply on behalf of the applicant.
- We provide the U.S. FDA inspection team with the authority to neglect further complexities.
- We have certified U.S. Agents through which we can provide you with the Certification in the minimum time.
- We also provide various other licenses and their compliances with U.S. FDA Certification.
- We have experts who will guide you in all the required test conducts, other registration required, etc.
- We provide a quick reminder before the certification is expired.
- We provide you updates regarding the status of the Certification applied at every stage.
Why Bizadvisors?
The Bizadvisors have great facility for the client’s business upbringing and the following are some reasons one should choose Bizadvisors:
.png)
- We minimize legal requirements.
- Our clients can also keep track of the progress on our platform at any moment.
- Our knowledgeable professionals are here to answer any queries you have.
- We will make sure that your interactions with professionals are pleasant and smooth.
- We always try our best to make our clients happy with the legal services we provide.
- We provide free legal advice.
- Our prices are transparent and reasonable.
- We deliver your work on time.
- We have a team of experts.
- We give money-back guarantees as well.
- 200+ CA and CS assisted us in our work.
- We give an option of easy and convenient EMIs.
U.S. FDA Certification is an essential requirement for all establishments that are engaged in the export of business commodities to the U.S. or other nations from India. It is a pre-requisite for all the products to be registered. The FDA Certification is an international certificate that assures the quality and standards of various goods and other products to be exported to the US. Obtaining it leads to various benefits for the applicant, its commodities, and the nation. Thus for expanding or running a business in the U.S. for food and drug export services, it is mandatory to be obtained.
Obtaining the U.S. FDA requires various testing of products, its proof, U.S. Agents, documents, and other procedures to be accomplished in the time specified. All these become cumbersome at some or other point in time. Thus it is advisable to hire a Professional who would assist in the process of U.S. FDA Certification and its compliances. We at Bizadvisors provide such facilities to our clients. Our Professionals have great knowledge regarding the U.S. FDA Certification and its requirements and compliances.
So, you can contact Bizadvisors to hire the services for U.S. FDA Certification and its compliances. Various other legal services are also provided by Bizadvisors. You can take a glance at it on our website. Do contact our team of professionals and experts to obtain successful U.S. FDA Certification at your doorstep.
Frequently Asked Questions
FDA Certification is a proof of document that certifies that the products exported from India to the U.S. are safe for consumption and use. The quality and standards are specified by the FDA Authorities and the same shall be compiled by the applicant. It ensures human health and safety in the U.S.
FDA established its office in India in the year 2008 in New Delhi. The office was established to certify the products that are safe for consumption and use to export to the U.S.
To acquire the U.S. FDA Certification, the following documents are required to be uploaded by the applicant:
- Landing bill
- Airway bill
- Invoices issued by the entity
- Purchase order details
- Packing list details
- Growers list details
- Labeling copies details
- Proof indicating the product’s real owner
- Details showing the use of the product exporting
- Other related documents are requested to showcase.
The different phrases of the FDA are as follows:
- First, Discovery and Development
- Second, Research and Test
- Third, Clinical Research
- Drug Research and review
- FDA drug safety monitoring
The following are the activities of the FDA in India:
- Regular inspections of medical products and equipment that are exported to the US.
- The U.S. FDA also consults and builds relations with Indian regulatory authorities to maintain the required quality and standards of products exported.
- Providing training to the Indian employers and regulators for Indian pharmaceutical and foods manufacturing industries to maintain the quality and standards requirement of foods and drugs.
FDA approval is not required for the following products:
- Vaccines for animals
- Pet foods
- Veterinary drugs
- Veterinary devices, etc
The following are the benefits of U.S. FDA Certification In India:
- Products goodwill
- Customers Confidence
- Business expansion into other nations
- Raise multiple investments
- Development of business sectors
- Boost product efficiency
- Creating a huge network in the country
- Online procedure for filing
- One can reach us by visiting our official Bizadvisors site. You can call or send your queries to us on the site itself.
- We’ll revert to you as soon as possible and satisfy your related queries.
- Next, you need to submit the documents and information related to U.S. FDA Certification.
- Will provide you with the same as decided.
 9559179325
9559179325 9559179325
9559179325 9559179325
9559179325











