Overview of CDSCO Registration
In India sale, manufacturing, import, distribution e.t.c. of drugs have been governed and regulated by the Drugs and Cosmetics Act, 1940 and Drugs and Cosmetics Rules, 1945. Any substance which falls under the definition of drugs as per the above-mentioned Act and Rules required getting registration before importing such substance outside the country. As per the rules and regulations of our country CDSCO i.e. Central Drug Standard Control Organization is the one that gives CDSCO Registration. It is a National Regulatory Authority under the Goew drug or clinical trial of any drug cannot happen without the approval of the CDSCO. It laid down the standard of drugs and also keep eye on the quality of the drugs. It also coordinates the work of the State Drugs Controlvernment of India, Directorate of Health Service, and Ministry of Health and family welfare. Any n Organization. Drugs and Cosmetics Act, 1940 is also facilitated by the same organization for its better implementation.
Any organization that is involved in the import, export, or manufacturing of drugs and cosmetics, definitely does Research and Development regarding the same. And this is done so that they can examine the BA that is Bioavailability and BE that is Bioequivalence of the drugs and cosmetics. For all this, they have to take one registration that is known as CDSCO Registration. This registration has been obtained through CDSCO online portal.
Who is eligible for CDSCO Registration?
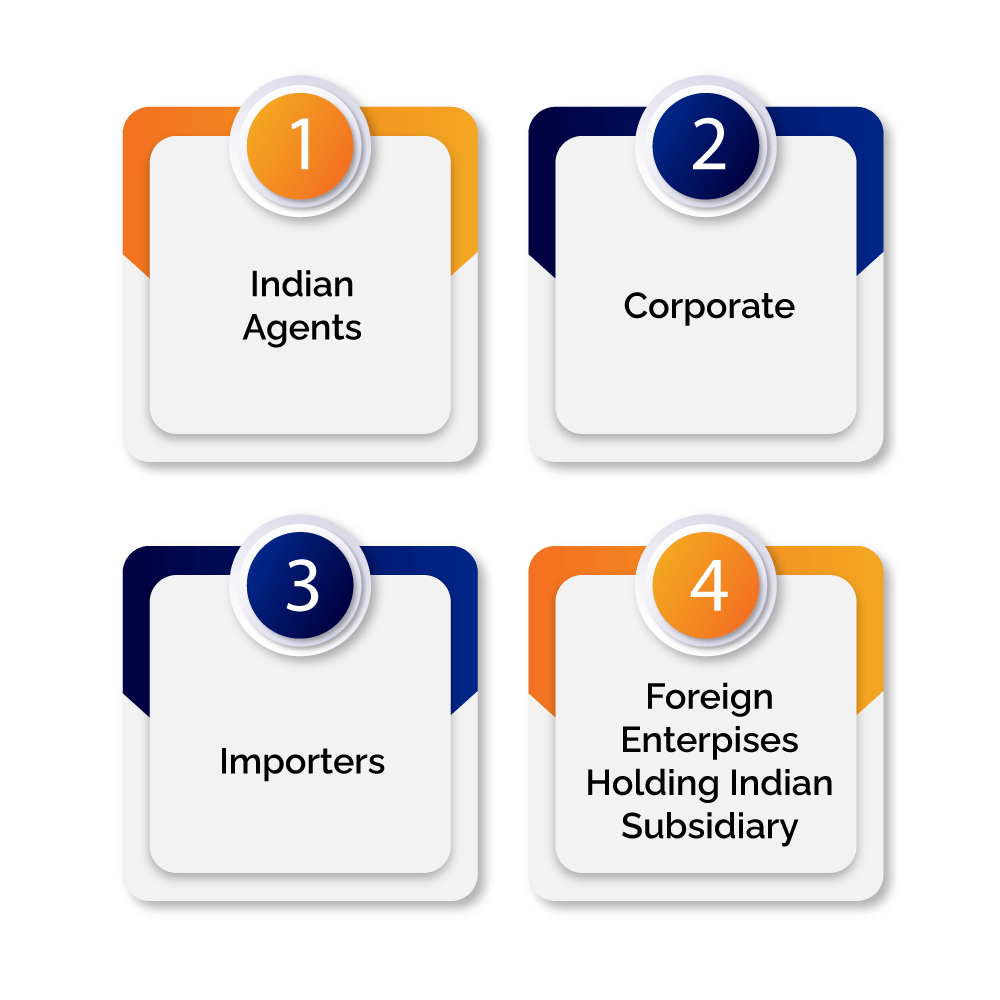
Given below is the list of applicants who can apply for CDSCO Registration-
CDSCO Registration Purpose
CDSCO Registration as per the rules and regulations is for many purposes, some of them are given below-
- To get the Test License done;
- Dual Use No Objection Certificate for the traders;
- For the purpose of registering as a Blood Bank that is known as Blood Bank Registration
- Then also for the purpose of getting Cosmetics Registration;
- BA that is Bioavailability or is that is Bioequivalence approved sites;
- Import of the drugs and also for the manufacturing of the drugs;
- For the purpose of registering Ethics Committee;
- Research and Development Organization formulation;
- No Objection Certificate for export in connection with any particular Zone;
- The registration of Blood Products and that registration is known as Blood Product Registration.
Benefits of CDSCO Registration
CDSCO Registration has been useful in many ways some of them are given below-
- Make sure the drugs which are manufacturing and imported are safe and good for us.
- Manage the quality of drugs and cosmetics in the market.
- Make the drug and cosmetic markets safe for the users.
- Increase the goodwill of the manufacturer and the importer in the market.
- Increase the credibility of the manufacturer and importer in the market.
- Built trust and increased reliability.
- Government can easily keep an eye on the manufacturer and importer’s activities.
- It will definitely make the drug and cosmetics market clean and safe for the customers.
Medical Devices Classification
India is one of the major countries whose import business in Medical Devices is great in comparison with other countries. India offers a global market for trade in Medical Devices. For such kind of market, it is very necessary to have a regulatory body. CDSCO is the organization that looks after drugs and cosmetics-related things in India with the help of IMDR 2017 guidelines issued by the CDSCO. Medical Devices have been classified mainly into two categories which are given below-
1.Notified Medical Devices
There are certain medical devices as per the Medical Rules, 2017 whose certification is mandatory. As per the same rule, there is a total of 37 such products for which it is compulsory to get this CDSCO Certification, drugs and cosmetics will also come under the same category. Classification of Notified Medical Devices-
2.Non-Notified Medical Devices
After the new amendment which is issued by the CDSCO, there are total of 313 products for which it is necessary to get CDSCO Certification. Although these products came under Non-Notified Medical Devices their certification is necessary. Obtain the approval for Non-Notified Medical Devices with the help of the procedure given below-
.png)
Notified Bodies for Medical Devices in India
India is a federal nation that we are divided into two parts that are state and central. This is completely correct as well because it is very difficult to manage both states as well as central organizations simultaneously. Hence our notified bodies for Medical Devices have been divided into two parts, which are given below-
1.State Licensing Authority
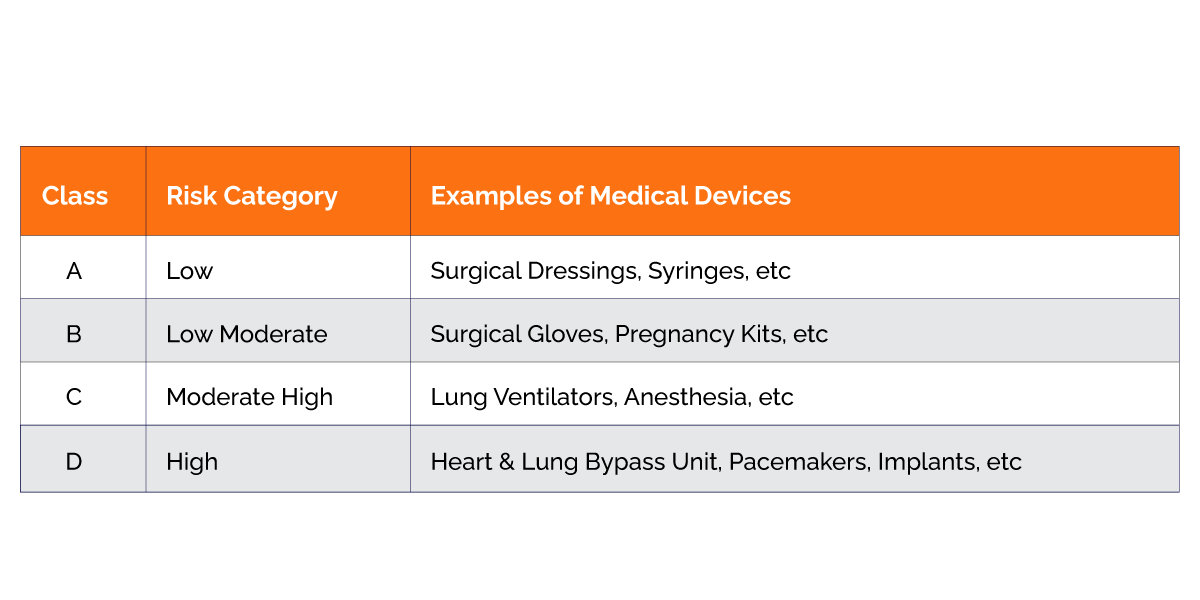
SLA is the body that has the responsibility to provide manufacturing, loan, and wholesale licenses to the Medical Devices under Class-A and Class-B categories. It also looks after the Quality Management System of the Medical Devices under the above-mentioned class.2.Central Licensing Authority
CLA is the body that has the responsibility to provide manufacturing, loan, and wholesale licenses to the Medical Devices under Class-C and Class-D categories. It also looks after the Quality Management System of the Medical Devices under the above-mentioned Class-C and Class-D.
Documents Required for CDSCO Registration in India
Although getting CDSCO Registration is an online process, you need to upload certain documents without which it is impossible to get this registration. The list of such documents which are compulsory or mandatory for the CDSCO Registration is given below-- Identity proof of the applicant who wants CDSCO Registration
- Address proof of the applicant who wants CDSCO Registration
- Upload the copy of BE or BA Site Registrations
- Undertaking allotted by a Government Authority regarding the Medical Device
- Wholesale License in case the business is in wholesale
- Manufacturing License in the case of Manufacturing
- Import of Blood Product Registration
- Drugs or Test License Registration.
Procedure for Obtaining CDSCO Registration
CDSCO Registration is an online registration procedure. There are many formalities that need to be done while registering. All information should be filled in carefully and duly checked. A single mistake can result in the rejection of your registration; hence it is advisable to take help from experts to make your work done on time. Given below are the steps to follow for obtaining CDSCO Registration-
- Visit the official CDSCO Registration portal.
- Then for the purpose of registering, you have to go to the “Registration Purpose page”
- After following the above steps now click on submit.
- Then you need to open the “Application Registration page”.
- Fill in all the information asked by them very carefully and check it once you have done it.
- After completing the above formalities now you have to fill out the registered Indian Address Form.
- Submit the application form and before submitting it checks it once again carefully.
- If everything is fine and all the information asked by the authorities is fulfilled correctly, then you will get a confirmation link to your registered email id, for the purpose of verification.
- Click on the same link which has been sent on your Registered e-mail id and activate your account on the CDSCO official portal.
- Now the application will be sent to the concerned authority for their approval.
- If the application for CDSCO Registration is approved then you will get the approval through your registered email.
- Once you get the approval mail, your CDSCO Registration will be completed and in a few days, you will get the Registration Certificate.
Different Divisions of CDSCO
When we talk about CDSCO, its divisions cannot be left out. Hence we will discuss its division below one-by-one-
1.Cosmetics
As per the Drugs & Cosmetics Act, a Cosmetic’s definition includes any item that is deliberately pat, sprayed, used, or applied to the human body or any part of the human body for the purpose of decorating, cleaning, glowing, or changing the appearance. It also includes any item intended for use as a cosmetic component.
Under the Act & Rules made there, the manufacturing of cosmetics is controlled by the State Licensing Authority which has been assigned by the concerned State Governments. On the other hand, when it comes to importing cosmetics, it is governed by a registration system through the authority appointed by the Central Government.
2.DCC-DTAB
DCC-DTAB gives suggestions on the matters raised out of the administration of the Act & Rules regarding drugs and cosmetics. DCC-DTAB also organizes the meeting for effective checking of the rules and observing the rules.
3.BA/BE
BA stands for Bioavailability, similarly BE that is stand for Bioequivalence is defined precisely here. BA is the respective amount of drug that reaches the intrinsic circulation and is able to have an active effect on the body. On the other hand, BE means two drugs whose function is to provide the same in all intent in equivalent proportions.
4.Import and Registration of Drugs
The import of rigs into India is governed and regulated under Chapter III of the Drugs and Cosmetics Act and Part IV of Drugs and Cosmetics Rules. The Applications for CDSCO Registration and also the Import License of drugs and cosmetics are processed as per the Drugs & Cosmetics Rules.
5.New Drugs
Taking care of the safety & efficacy of the drug and cosmetics products that are used on humans is very important before the drug or cosmetic product can consent for manufacturing and imported into the country. New Drugs as defined under Drugs & Cosmetics Rules, need to be examined before they got supplied in the market, very strictly after all health of the public at large depends on such drugs and cosmetics.
6.Medical Devices and Diagnostics
In India, presently only some notified Medical Devices are regulated and governed under the Drugs and Cosmetics Act and Rules. Substances such as incision dressing stapes,sutures, bandages, ligatures, blood & its component collection bag with/without anticoagulant are some examples of Medical Devices. Some substances are also used as artificial insemination recognition and some substances comprise prophylactic techniques, intrauterine devices, antiseptic & insect killers. All such substances are also governed and regulated by the Acts mentioned above and Rules as they all fall under the category of Medical Devices.
7.Biological
Biological products are medical products either directly or indirectly, and also most of the biological products available on earth are made from a variety of natural sources themselves. Our whole Ayurveda depends on nature for the medicines and the other treatment.
8.Vaccines
Human organs, cells, tissue, etc are used for transplantation, and different types of therapies, while doing Blood donor tests, some allergic extracts from plants are used for treatment & diagnosis of different types of diseases and work as a cure for them.Why you should choose Bizadvisors?
BizAdvisors is one of the platforms that work together to meet all of your legal and financial needs while also connecting you with dependable specialists. Yes, our clients are happy with the legal services we provide. They have continually regarded us well and provided regular updates because of our focus on minimizing legal requirements. Our clients can also keep track of the progress on our platform at any moment. Our knowledgeable professionals are here to answer any queries concerning the CDSCO Registration. BizAdvisors will make sure that your interactions with professionals are pleasant and smooth. Following are the reasons one should choose Bizadvisors-
- BizAdvisors is one of the many platforms which coordinate to fulfill all your legal requirements.
- It connects you with a team of expert professionals who can help you in every possible way.
- Its focus is on simplifying the legal requirements for the client.
- If you have any questions regarding CDSCO Registration we are just one phone call away.
- We have a very dedicated team that is ready to help you and guide you.
- Our mission is to create a hustle-free and easy-to-use system for the concerned consumers of our services.
- We give you reliability and trust.
- We make sure that we will provide you with the best services and can satisfy you with our quality work.
India has a big market for drugs and cosmetics. It not only manufactures drugs and cosmetics but also imports and export the same. Hence it is very important to govern or regulate the activity of the Medical Devices market. This market involves the health and safety of the public at large, after all these drugs and cosmetics will be consumed or used by the people at last. For the purpose of safety and security, CDSCO is an organization that controls and regulates the activity of the Medical Devices market by providing CDSCO Registration. Although the process of getting this registration is an online process, there are many formalities related to this which need to be done. Hence expert advice is always suggested while getting this license because even a small mistake can waste your time and money.
FAQs related to CDSCO Registration
For the purpose of safety and security CDSCO is an organization that controls and regulates the activity of the Medical Devices market by providing CDSCO Registration
Given below is the list of applicants who can apply for CDSCO Registration-
- Indian Agents
- Corporate
- Importers
- Foreign Enterprises Holding Indian Subsidiary
The procedure to get CDSCO Registration is given below-
- Visit CDSCO's official portal.
- Fill out the application form.
- Upload the documents.
- Authorities will verify the application.
- After valid verification one will get the CDSCO Registration.
Following are the divisions of CDSCO-
- Cosmetics
- DCC-DTAB
- BA/BE
- Import and Registration of Drugs
- New Drugs
- Medical Devices and Diagnostics
- Biological
- Vaccines
Permission taken by the government for the manufacturing, selling, processing, distribution, and sale of the Medical Devices which include different types of drugs and cosmetics, in the market is known as CDSCO Registration. It will help the government to make the Medical Devices market safe for the public as it directly affect public health.
Following is the list of advantages of CDSCO Registration-
- Reliability
- Safety
- Security
- Goodwill
- Trust etc
 9559179325
9559179325 9559179325
9559179325 9559179325
9559179325











