Brief of Drug License
In India, it is obligatory for every person who wants to start a Medicine or Cosmetic business to obtain Drug License. The term “Drug” denotes a substance that is used for curing diseases. That means it directly guarantees the well-being of a human being. Further, for the purpose of manufacturing medicines, different drugs are used in a specified ratio.
Features of Drug License
The features of a Drug License are as follows:
- The term “Drug” includes Ayurvedic Medicines, Homeopathy, Allopathic Drugs, and Unani;
- The process of obtaining Drug Registration is location-specific in nature;
- If in case a business entity has operating units in two states or more states, then it needs to obtain a separate drug license for each state;
- The Central Standard Control Organization and State Standard Control Organization have the authority to grant Drug License in India;
Different Types of Drug License
The Different types of Drug License are as follows:
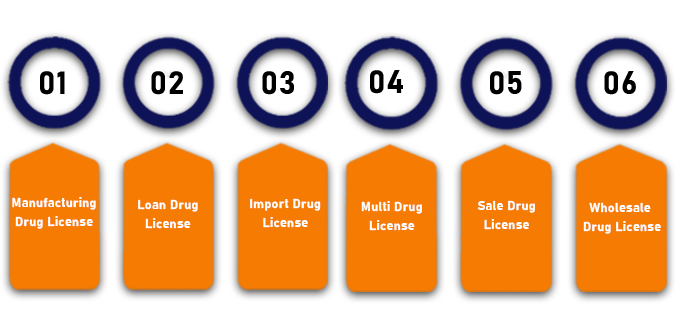
Manufacturing Drug License
All the manufacturers who are dealing with ayurvedic, allopathic, cosmetics, medicines, etc., as specified under the Drugs and Cosmetics Act 1940, needs to obtain Manufacturing Drug License. Further, it a state license and is issued by the respective state government where such a unit is located.
Loan Drug License
Any manufacturer who does not possess any land on his/ her own, but wants to manufacture drugs by using his/ her brand name on the land needs to obtain Loan Drug License.
Import Drug License
An Import Drug License is required by every dealer who imports the products for manufacturing drugs or is engaged in the business of importing drugs in India.
Multi Drug License
A Multi Drug License is needed by the businesses which are operating in more than one state.
Sale Drug License
All the business entities involved in the retail or wholesale distribution of drugs in India need to obtain Sale Drug License.
Wholesale Drug License
All the individuals who are engaged in the Wholesale business of pharmaceuticals need to obtain wholesale drug license from the CDSCO. Further, the minimum requirements for this license are as follows:
- Degree or Diploma in Pharmacy;
- Minimum 1-year prior experience;
Retail Drug License
All the individuals who are either stand-alone pharmacists or are involved in the retail distribution of drugs need to obtain Retail Drug License. Further, this license is issued by the State Pharmacy Council.
Key Requirements for Obtaining a Drug License
The key requirements for obtaining a Drug License in India are as follows:
Premise Area
Any individual who wants to set up a Medical Store or a Retail Pharmacy needs to have a minimum area of 10 sq mtrs. However, it shall be relevant to state that in the case of a Retail Cum Wholesale Drug Business, the minimum area required will be 15 sq mtrs.
Storage Facility
An individual must ensure that the premise is equipped with proper storage facility, such as the refrigerator, cold storage facility, to store medicines.
Technical and Storage Staff
It is mandatory for a Retail Pharmacy Store to have an experienced and technical staff with complete knowledge regarding the same. Further, it is necessary for the Pharmacist to be qualified for obtaining a Drug License.
However, it shall be relevant to note that in the case of a Wholesale Drug License, the staff appointed needs to a graduate with 1-year experience or an undergraduate with 4 years’ experience,
Inspection of the Premises
Prior to the issuance of Drug License, a Drug Inspector, who is having jurisdiction, has the authority to visit the premises for inspection. Further, he shall have the power to verify the details furnished, measure the premises, and interview the competent person.
Different Forms for Drug License
The different forms for Drug License are as follows:
|
FORM |
Types of Drugs |
|
FORM 20 |
Allopathic Drugs; |
|
FORM 20- A |
Restricted Allopathic Drugs; |
|
FORM 20- B |
Wholesale of Allopathic Medicines; |
|
FORM 20-C |
Retail of Homoeopathic Medicines; |
|
FORM 21 |
Allopathic drugs mentioned in the Schedule C & C (1); |
|
FORM 21- B |
Wholesale of Allopathic Medicines specified in the Schedule C & C (1); |
|
FORM 21- A |
Retail sale of the Restricted Allopathic Medicines specified in the Schedule C(I); |
|
FORM 20- F |
Retail Sale of the Drugs specified in the Schedule X; |
|
FORM 20- G |
Wholesale of the Drugs Specified in the Schedule X; |
Documents Required for Obtaining Drug License
The documents required for obtaining Drug License are as follows:
- Application Form;
- A copy of the Challan;
- PAN Card, Driving License, Passport, Voter ID, etc. as ID proof;
- In case of Partnership Firm, a copy of Partnership Deed;
- Memorandum of Association;
- Articles of Association;
- Certificate of Incorporation;
- A copy of the Site Plan for premises;
- A copy of the Key Plan of the premises.
- CTC of the BR (Board Resolution) for authorising the company to obtain the license;
- Appointment Letter for the person being authorised by the company;
- A copy of the Sale Deed or Property papers (In the case of an owned property);
- A copy of the Lease Deed or Rent Agreement (In the case of rented property);
- Proof of Storage Availability, such as the Cold Storage and Refrigerator, in the form Invoices;
- Affidavit concerning the compliance of MPD 2021;
Additional Documents
Registered Pharmacist
In the case of Retail Sale, the additional documents required are as follows:
- Proof of Qualification (Certificate of Final Degree or Provisional Certificate, together with the Marksheet);
- Registration from the State Pharmacy Council;
- A copy of the Bio-data;
- A copy of the Appointment Letter;
Competent Person
In the case of Wholesale License, the additional documents required are as follows:
- Proof of Qualification (Certificate of Final Degree or Provisional Certificate, together with the Marksheet);
- A copy of the Bio-data;
- A copy of the Appointment Letter;
- A copy of the Minimum 1 year Experience Certificate;
Procedure for Obtaining Drug License in India
The steps involved in the procedure for obtaining Drug License in India are as follows:
Login Credentials
In the first step, the applicant needs to submit a request letter, together with the firm details to obtain a User ID and Password.
Prepare Documents
In the next step, the applicant requires to prepare documents, which includes Site Plan, Key Plan and various other declarations and affidavits prescribed by law.
Filing of Application
Now, the applicant needs to file an online application for obtaining Drug License, together with the fee prescribed by the government. Further, all the documents required must be scanned in 100 DPI, black and white, and should be uploaded at the time of filing the application for Drug License as well.
Visit by Drug Inspector
Once the online application for the issuance of Drug License is filed, the same will then marked for inspection.
The term “inspection” denotes that the Drug Inspector will personally visit the concerned shop in order to verify the documents filed, and will check the correctness of all the details submitted as well.
Issuance of License
After the successful completion of the process of Inspection and verification, the Controller of Drugs will then issue the Drug License to the said applicant.
Time Period Required for Drug License
Normally, a period of 45 to 50 business working days is required for the issuance of Drug License.
Why Choose Us

Free Legal Advice

Transparent Pricing
On Time Delivery
Expert Team

Money Back Guarantee

200+ CA/CS Assisted

Lowest Fees

Easy EMIs
Frequently Asked Questions
The term “Drug License” signifies the permission given by the government to deal with medicines, drugs and cosmetics.
Only the State Government has the authority to grant Drug License to the applicant.
 9559179325
9559179325 9559179325
9559179325 9559179325
9559179325











